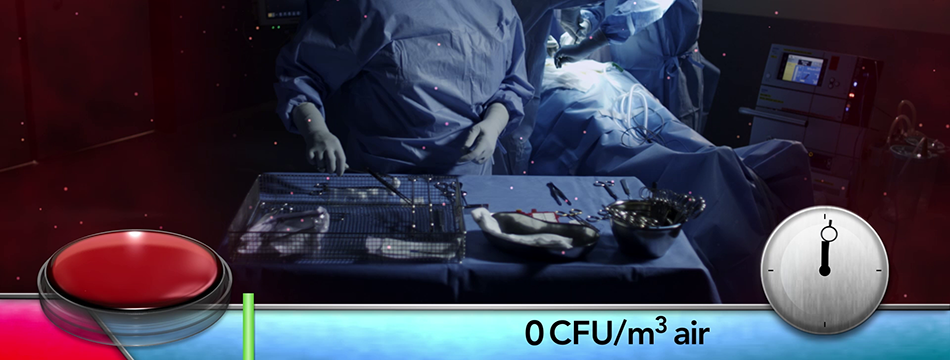
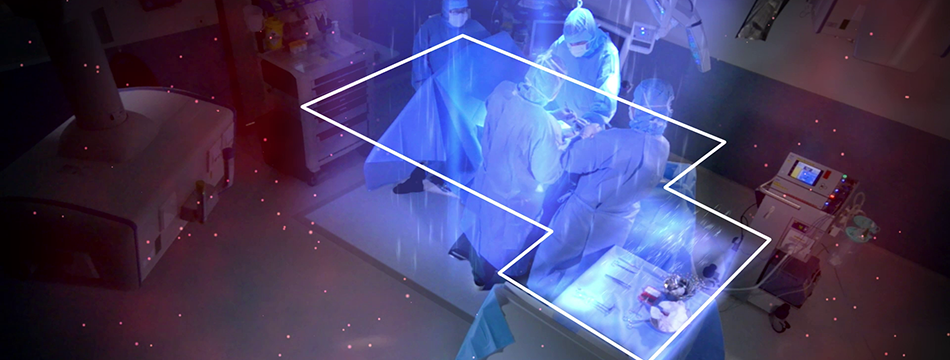
Operio was tested in a clinical setting for intravitreal eye injections in October 2015 in a Swedish University Hospital. Measurements where made near the surgical site and over the instruments where the results demonstrated a statistically significant reduction in CFU levels as well as particulates. The CFU value is based on 31 samples from 23 patients. As a mean value, the Operio had a CFU counting of 0.4. For particulate counting, the Operio had a mean value of 1.1 particles of 0.5 μm/f³. For more information, request our report “Operio at intravitreal injections”.
Published clinical papers
A vast amout of studies discuss the effect of clean air in the OR. Toul Meditech has compiled a list with published studies that discuss the effect of clean air in the operiation room that are of particular interest in respect to our devices.
|
Year |
Name of publication |
Relevance |
| 2022 | Giuseppe Scarpa, Francesca Urba, Margherita Scarpa, Stefano Formentini and Andrea Beccastrini. Intravitreal Injections during the COVID-19 Outbreak in Northern Italy: An Innovative Approach for a High Quality and Safe Treatment. European Journal of Ophthalmology. | This observational study was conducted which investigated the outcomes of IVIs performed in an ophthalmologist’s office using a mobile laminar flow unit, the Operio mobile (Toul Meditech, Operio®) versus an operating room setting. |
| 2018 | A.-C. von Vogelsang, P. Förander, M. Arvidsson, P. Löwenhielm. Effect of mobile laminar airflow units on airborne bacterial contamination during neurosurgical procedures. Journal of Hospital Infection. | The study was conducted at a neurosurgical operating suite at Karolinska University Hospital in Stockholm, and a quasi-experimental design was used, shifting MLAF units monthly. Where the study mentions MLAF this means SteriStay instrument table and Operio clean air flow unit. |
|
2016 |
Possible contamination of instruments in the operating theatre during implantation of hip and knee endoprostheses. Georg Thieme Verlag KG. |
The abstract describes a comparative study between SteriStay instrument table supplied with clean air from Toul Meditech and a conventional instrument table without clean air. This prospective controlled study was conducted to demonstrate the effect on contamination by pathogens of instrument tables with integrated horizontal flow, compared to conventional tables. |
|
2011 |
Dr Sossai, G. Dagnino, F.Sanguineti, F. Franchin. Mobile laminar air flow screen for additional operating room ventilation: reduction of intraoperative bacterial contamination during total knee arthroplasty. J Orthopaed Tramuatol (2011) 12:207-211. |
This study was carried out on a previous model from Toul Meditech (Toul 400) which has the same technology as Operio. The study was performed during an authentic knee operation to verify the efficiency of a clean airflow over the surgical wound. |
|
2010 |
Friberg S. Umeå University medical, Assessment of horizontal laminar airflow instrument table for additional ultraclean space during surgery: Journal of Hospital Infection 76 (2010) p 243-246 |
This study was carried out on a previous model from Toul Meditech (Toul 300) which has the same technology as the instrument table SteriStay. This study was performed during an authentic knee operation using an instrument table with a sterile shield. The study supports the fundamental hypothesis that also the instruments should be protected during surgery with clean air. |
|
2003 |
Friberg S. Umeå University. The addition of ultra-clean exponential laminar airflow screen to conventional operating room ventilation reduces bacterial contamination to operating box levels. Journal of Hospital Infection. Elsevier. |
This study was carried out on a previous model from Toul Meditech (Toul 400) which has the same technology as Operio. The study was performed during 60 standardized operations for groin hernia to verify the efficiency of a clean airflow over the surgical wound. |
|
2001 |
Friberg B. Umeå University. Mobile zoned/exponential LAF screen – a promising new concept in ultra-clean air technology for additional operating room ventilation. Journal of Hospital Infection (2001) submitted may 2001. |
This study was performed as a sham operation using the earlier types of Toul Meditech’s air cleaning device to verify the efficiency of a clean airflow over the surgical field. Where the abstracts refer to a device named “Mobile zoned/exponential LAF screen” this is the initial model which was the first prototype where Toul Meditech air cleaning technology was used. |
|
1983 |
Lidwell OM et al. Airborne contamination of wounds in joint replacement operations, the relation to sepsis rate. Journal of Hospital Infections 1983b; 4: 11-131 |
This fundamental study verifies the importance of clean air within surgical procedures in general. |
|
1982 |
Whyte W et al. The importance of airborne bacterial contamination of wounds. Journal of Hospital Infections 1982; 3: 123- 135 |
This fundamental study verifies the importance of clean air within surgical procedures in general. |
FAQ about Operio
Our customers usually ask questions about positioning and ventilation. Here is a summary of typical questions regarding the use of the Air Flow Unit Operio.
Do I need to take special precautions if exposed to MRSA?
When MRSA occurs, it is advised that all exterior surfaces of Operio are cleaned with isopropyl alcohol (or alcohol not less than 70%) and to change the LAF shield. The HEPA filter does not need to be replaced. MRSA is mainly a contact related contamination.
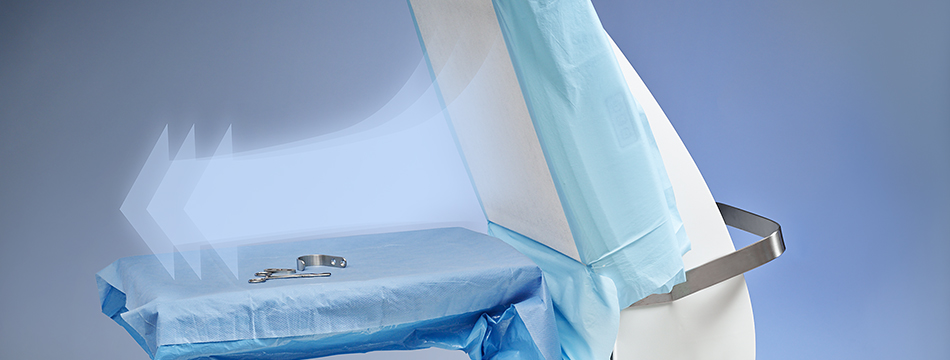
Does Operio disturb the regular ventilation in the OR?
No, the airflow is locally distributed over the surgical site and instruments only and has no impact on the regular ventilation system.
How shall I best position Operio?
Place Operio in a straight position to where you need the clean air to be distributed. A good distance is 30 - 45 inches / 75 – 115 cm.
The effectiveness of the airflow declines after 47 inch/120 cm, so make sure that the surgical site is not beyond this distance. The width of the airflow is 20" (50 cm) so make sure that the surgical site is within this specified width, which is the same width as the hood.
Can I use Operio as an instrument table?
Yes, you can use the Operio with the attached tray as a small instrument table or you can extend the clean airflow area by adding an extra instrument table. You can also use your own instrument table in front of the Operio if the tray is detached. When using your own instrument table, place the table at the same height as the tray. If using a tray and an instrument table, depending on the length, it may not be enough airflow also for the surgical site. So keep in mind not to block the airflow or placing the patient to far away.
Where shall I best place the instruments on the tray?
Objects of a total weight up to 11 lb (5kg) can be placed on the tray. Spread them evenly with the smallest objects closest to the airflow outlet. Avoid placing larger objects on the tray since that may block the clean airflow.
How far does the clean airflow reach?
47 inch/120 cm.
Is Operio noisy?
No, only 47 dB - which is equal to the sound of a computer fan. The noise comes from the built in fan, which is necessary for the airflow to have a certain speed.
How does the airflow from Operio affect the ambient environment?
It affects only in a positive way since 400 m3 of air is recirculated and cleaned by the HEPA filter every hour. With the airflow on, it keeps cleaning the ambient air in the room. However, the intention is not to clean all air in a room, just the local area.
How about frequent opening of doors?
It doesn’t affect the airflow from Operio since it is locally distributed over the surgical site. However, frequent opening of doors is never recommended since it stirs up particles and has a negative impact on air quality in general.
Is it a risk that I am in the way of the airflow?
You should try not to block the airflow or stand in the way for any longer period. Normal movement of hands and arms are no problem. Think of it as if you would stand in front of a lamp making a shadow.
How shall Operio be placed during preparation?
Turn on the airflow and place the instruments on the tray in the same way as you normally would do on an instrument table. The airflow over the tray is always optimally directed even if the position of the unit is shifted.
If I already have prepared Operio with instruments and draping, what shall I do to keep it ready for the procedure?
Leave the airflow on. If Operio needs to be moved any longer distance, cover the instruments with drapes, unplug Operio, move the unit, start the unit and the airflow again. Remove the covering drapes from the tray.
Why is it necessary to have a sterile shield?
Operio is a medical device and needs to fulfil regulatory requirements. The sterile shield act as a physical barrier that protects the patient and staff from contamination. The sterile shield also forms the airflow to a perfect air column without turbulence.
Does Operio require a specific draping on the tray?
No, you can use regular OR drapings. The sterile shield is intended to protect the hood and airflow outlet.
Does the surgical staff need specific OR clothing when using Operio?
No, you can use your regular OR suits.
What about power failure, what happens?
The airflow needs to be restarted in the case of a power failure. If the power is missing for more than 15 minutes the sterile shield needs to be replaced. If there are sterile instruments on the tray they will not be protected if the power is off. To protect the instruments, you can cover them with a sterile drape while the power is off.
How clean is the airflow and how can it be verified?
Air cleanliness can be measured by using an active air sampler and agar plates which can be cultivated in a test laboratory. The air cleanliness should be under 5 cfu/m3 if sampled in the clean airflow and under normal use. Clinical tests have been carried out with results < 1 CFU /m3.
By using a particle counter the cleanliness of the air can also be measured. When there are no or few particles, there is no bacteria. This is the easiest way to verify.
What kind of maintenance is required?
The HEPA filter should be replaced once a year or every 2000 hours and a functional test shall also be carried out. If Operio is used longer than 2000 hours, the service indicator will be lit reminding the user that service is due. The device can still be used without replacing the HEPA filter, but it is on the user’s responsibility and the service lamp will stay on until it has been replaced.
Abstracts
To the right you will find some of our technical reports, please contact Toul Meditech AB for further info.
FAQ - Questions about SteriStay
Our customers usually ask questions about positioning and ventilation. Here is a summary of typical questions regarding the use of the instrument table SteriStay.
Do I need to take special precautions if exposed to MRSA?
When MRSA occurs, it is advised that all exterior surfaces of SteriStay are cleaned with isopropyl alcohol (or alcohol not less than 70%) and to change the LAF shield. The HEPA filter does not need to be replaced. MRSA is mainly a contact related contamination.
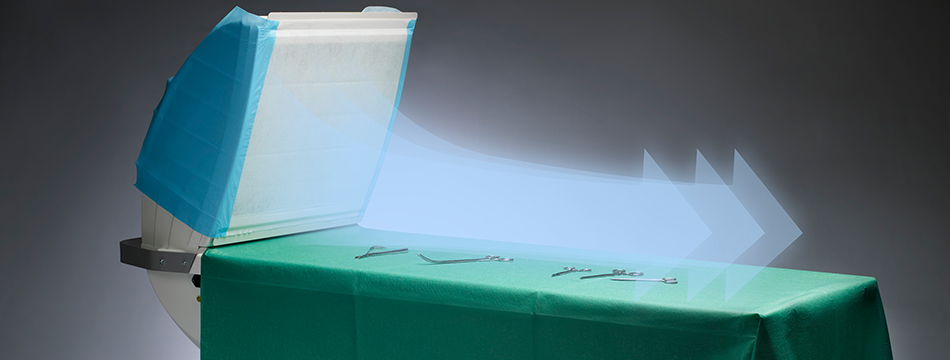
Does SteriStay disturb the regular ventilation in the OR?
No, the airflow is locally distributed over the instrument table only and has no impact on the regular ventilation system.
How shall I best position SteriStay in the OR?
Place SteriStay in the same way as you normally place your instrument table. If you need more than one table keep in mind that the airflows shouldn’t interfere.
Is SteriStay noisy?
No, only 47 dB - which is equal to the sound of a computer fan.
How does the airflow from SteriStay affect the ambient environment?
It affects only in a positive way since 400 m3 of air is recirculated and cleaned by the HEPA filter every hour.
How about frequent opening of doors in the OR?
It doesn’t affect the airflow from SteriStay since it is locally distributed over the instrument table. However, frequent opening of doors is never recommended in an OR since it stirs up particles and has a negative impact on air quality in general.
Is it a risk that I am in the way of the airflow?
No, not normally during preparation or use (hands and arms are no problem).
How shall SteriStay be placed during preparation?
Place the instrument table in the same way as you normally would do. The integrated airflow is always optimally directed even if the table is shifted.
If I already have prepared SteriStay with instruments and draping, what shall I do to keep it ready for the OR?
Leave the airflow on. If the instrument table needs to be moved any longer distance, cover the instruments with drapes, unplug SteriStay, move the instrument table, start the unit and airflow again. Remove the drapes from the instrument table.
Why is it necessary to have a sterile shield?
SteriStay is a medical device and needs to fulfil the regulatory requirements. The sterile barrier protects the patient from contamination. The sterile shield also forms the airflow to a perfect air column without turbulence.
Does SteriStay require a specific draping?
No, you can use regular drapings used in OR. The sterile shield also protects the hood.
How long can SteriStay remain turned off before a new sterile shield is needed?
15 minutes.
Does the OR staff need specific OR clothing when using SteriStay?
No, you can use your regular OR suits.
Where shall I best place the instruments on SteriStay?
Large objects and tall equipment shall be placed at the far end of the table not to disturb the airflow.
How far does the clean airflow reach?
The length of the table.
What about power failure, what happens?
The airflow needs to be restarted in the case of a power failure. If the power is missing for more than 15 minutes the sterile shield needs to be replaced.
How clean is the airflow and how can it be verified?
Under 5 cfu/m3 air. By using a particle counter the cleanliness of the air can be measured. When there are no particles, there are no bacteria.
But how do we protect the patient?
By using SteriStay 70% of the risk for air contamination is reduced wether the patient is protected or not. Toul Meditech also has devices that fully protect the patient and the wound site. Please see under Products.